High Quality Threat Administration Qrm: A Complete Guide For Excellence
Regulatory tendencies and present regulatory activity are additionally indicators offering insight into predictive dangers of regulatory audits. This course of ought to be considered in a holistic, proactive, and systematic manner, that means that QRM should be Software Сonfiguration Management an integral part of the pharmaceutical quality system, centered on identifying and evaluating potential dangers throughout the product lifecycle. Being proactive involves anticipating and addressing potential dangers earlier than they happen, while a scientific strategy requires a structured and constant methodology capable of figuring out, assessing, controlling, and speaking dangers. Risk management must also think about the continual monitoring and reassessment of recognized risks to guarantee that the danger management strategies stay effective. Quality danger administration is a systematic, risk-based strategy to quality administration.
- Risk administration actions ought to be performed by subject material consultants and key stakeholders at acceptable phases of a product’s lifecycle.
- The organization’s production engineers recognized a possible threat of their manufacturing process.
- Managing danger successfully could be a important challenge, significantly when the QRM process is not systematically organized all through the company.
- It additionally allows for traceability, which is crucial for root cause evaluation and for demonstrating compliance with rules.
- Despite being referred to as « simple instruments, » these provide a method more sophisticated approach than basic brainstorming by enabling cause-and-effect risk evaluation.
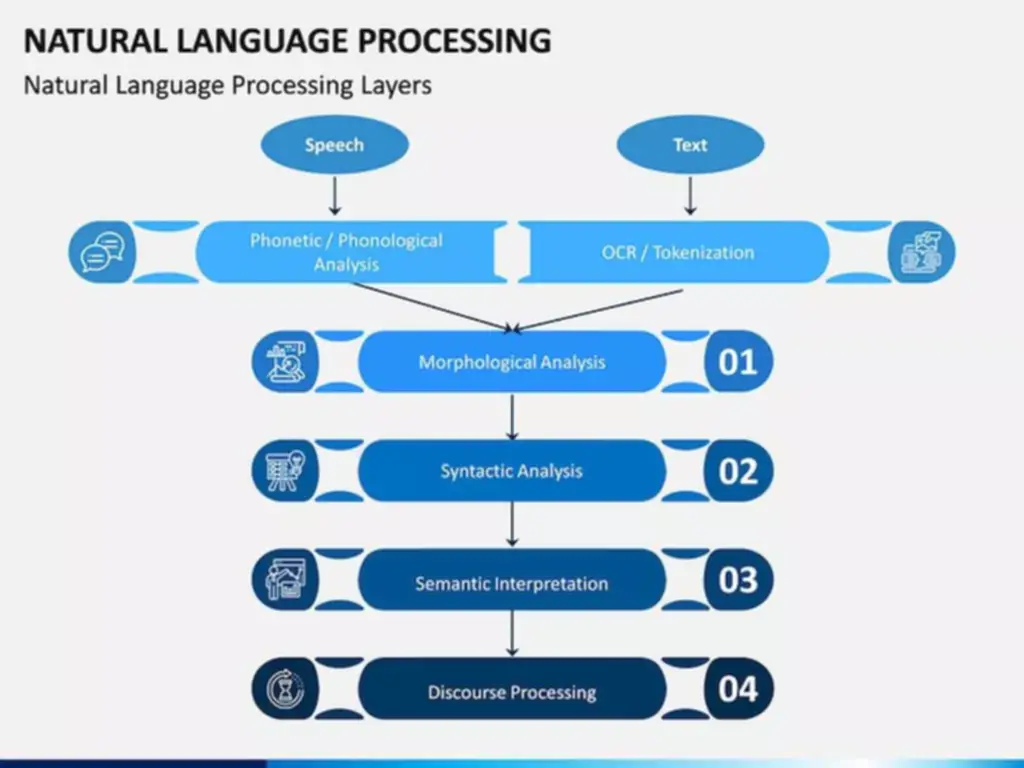
Holistic Identification Of Risks
That’s as a result of quality risks QA stakeholders are too dependent on high-profile outages to serve as a reminder of software program risk. In quality assurance (QA), threat is all concerning the « what ifs » that could negatively affect our future. It’s a combine of how probably one thing dangerous is to happen and how a lot it could throw us off track if it does. For anyone managing QA, staying on high of these risks is crucial to keep our software program in top shape. With the assistance of SafetyCulture (formerly iAuditor)’s Training, leaders, supervisors, and facilitators can create and deploy training packages targeted to the wants of the enterprise. They can even monitor course completion progress and make necessary follow-ups to ensure nobody misses training requirements for compliance.
A Mobile App For Managing Quality Risks
A test covering an software module with a high threat score has priority over checks that cover an utility module with a decrease threat score. Table I. Example of an optimized residing danger evaluation library for current good manufacturing follow (CGMP) operations. A robust biopharmaceutical QRM system recognizes that the method of risk identification is steady and dynamic, adjusting to changes in procedures, tools, materials, and the overall business environment.
What Is High Quality Risk Management ?
If there is an opportunity to forestall, mitigate, or eliminate a hazard, the step is taken into account a critical management level. Each member of this group will be answerable for coordinating high quality threat management across varied functions and departments in the firm. In our career, it’s important we follow ethical requirements and ensure that practitioners are impartial the place needed. In addition, a firm should obtain no less than annually a confirmation of compliance with independence from all personnel. Competence is the ability of an individual to carry out a job and goes past technical knowledge; it is the integration and application of that technical knowledge together with their skilled ethics, values and attitudes. Competence could be developed via quite lots of strategies and will depend upon the nature of your agency.

Failure Mode Effects Analysis (FMEA) is a systematic method of anticipating potential failures in a process, product, or design and mitigating the negative impression of those failures on consumers. Key elements of this tool are mechanism of failure, danger priority quantity (RPN), and follow-up corrective actions. Meanwhile, threat communication involves sharing info on the complete process with stakeholders who usually are not part of the QRM team. It also includes documenting the QRM team’s thought process at every step and the outcomes they have been able to achieve after finishing a step. Vaccine manufacturers, for instance, have displayed great agility by adapting their organisations from an enterprise, strategic and operational standpoint to produce Covid-19 vaccines.
Adapted from Training Programme for Q8/Q9/Q10 work merchandise developed by the ICH working group (Implementation of ICH Q8, Q9, Q10, by ICH (2010), retrieved from Adopting a risk-based mindset and integrating QRM ideas into day by day operations might require a cultural shift within the group. QRM could be utilized to all product life cycle levels, from product development to manufacturing, distribution, and post-market surveillance.

Plus, it could improve your project learning and innovation by identifying and addressing the foundation causes of high quality points or defects and applying greatest practices and lessons realized. Subjectivity may be compounded by groupthink as part of brainstorming activities—during hazard identification steps, for instance, and when likelihood rankings are being assigned. In addition, a lack of variety in risk-assessment teams can limit the breadth and effectiveness of risk-assessment exercises. As mentioned in ISO 31000,thirteen stakeholders form judgments about risks based on differences in values, needs, assumptions, issues, and so forth. As a end result, it could be difficult to reach settlement on the acceptability of a specific danger, or on the suitability of the course of action proposed to deal with that threat.
To achieve this, you probably can leverage numerous instruments, similar to a risk matrix for graphical representation of the dangers, a threat register to doc them, and danger evaluation software program to carry out quantitative or qualitative analysis. A risk matrix may help classify the risks into high, medium, or low categories, whereas a risk register can record their likelihood and impression ratings, causes and effects, and response methods and owners. Risk analysis software program can be utilized for Monte Carlo simulation, sensitivity analysis, or choice tree evaluation.
Example accountability project (RACI) matrix for high quality threat management (QRM) Master Plan. This can contain common critiques of the QRM system and a process for making adjustments to it. Scenario analysis or stress testing could possibly be used to evaluate and enhance the system’s adaptability.
To be certain that the vaccines are successful, the systemic dangers to high quality have to be addressed proactively from day one. If the vaccine does not work, it’s not the scientists or the researcher’s fault; it’s the management’s fault because, as Deming mentioned it finest, management is liable for high quality. The information and communication part is another space of how a response could vary from firm to agency. In a less complicated agency with fewer personnel and direct interaction between employees and leadership, casual communication may be enough. However, in a larger agency with many companions and staff, formal policies and procedures could additionally be required that specify how information ought to be identified, captured, processed, and maintained.
Most of the time, quality exists within the system in specialised however disconnected siloes. This may be achieved by utilizing a methods strategy and recognising QRM as part of Enterprise Risk Management (ERM). Russell Ackoff – one other ‘systems’ guru – tells us that systems can be understood by viewing and analysing them from totally different views. Unlike the standard goals stipulated in the standard, there are only a few specified responses.
The significance of QRM stems from its ability to make sure consistent product high quality, safeguard affected person security, and keep regulatory compliance. Quality danger administration (QRM) is an integral a half of good manufacturing practices (GMP) and essential for guaranteeing that pharmaceutical merchandise are protected, effective, and high-quality. ValGenesis delivers integrated and good solutions that support the digital transformation of the life sciences business. With a portfolio that covers the whole product lifecycle, ValGenesis has a digital or technical solution that brings worth to every step of your validation and manufacturing processes and their related activities.
If you are a small agency, the policy associated to growing personnel could also be so easy as on-the-job coaching and feedback and review of working papers. In a larger firm, the policy might require a senior staff member all the time critiques the work of a junior, and formal coaching applications are provided as staff transfer by way of every degree. And of course, if you are a sole practitioner, you must ensure you develop and preserve your personal competence to perform your position, maybe through the participation in professional development.
This doesn’t simply imply responding swiftly to emergencies—it means anticipating potential issues and managing them before they escalate. They need to be sharp and agile, catching issues as a part of QA testing efforts earlier than they turn into larger headaches. It’s about maintaining the software’s quality excessive, even when time is tight and the pressure is on. An instance of quality issues in construction is the use of lower-grade concrete than specified within the contract for the foundation of a building, which could lead to decreased structural integrity and longevity of the development. The commonest examples of quality defects in building embrace roof cracks, leaking plumbing, defective electrical systems.
Transform Your Business With AI Software Development Solutions https://www.globalcloudteam.com/ — be successful, be the first!


















